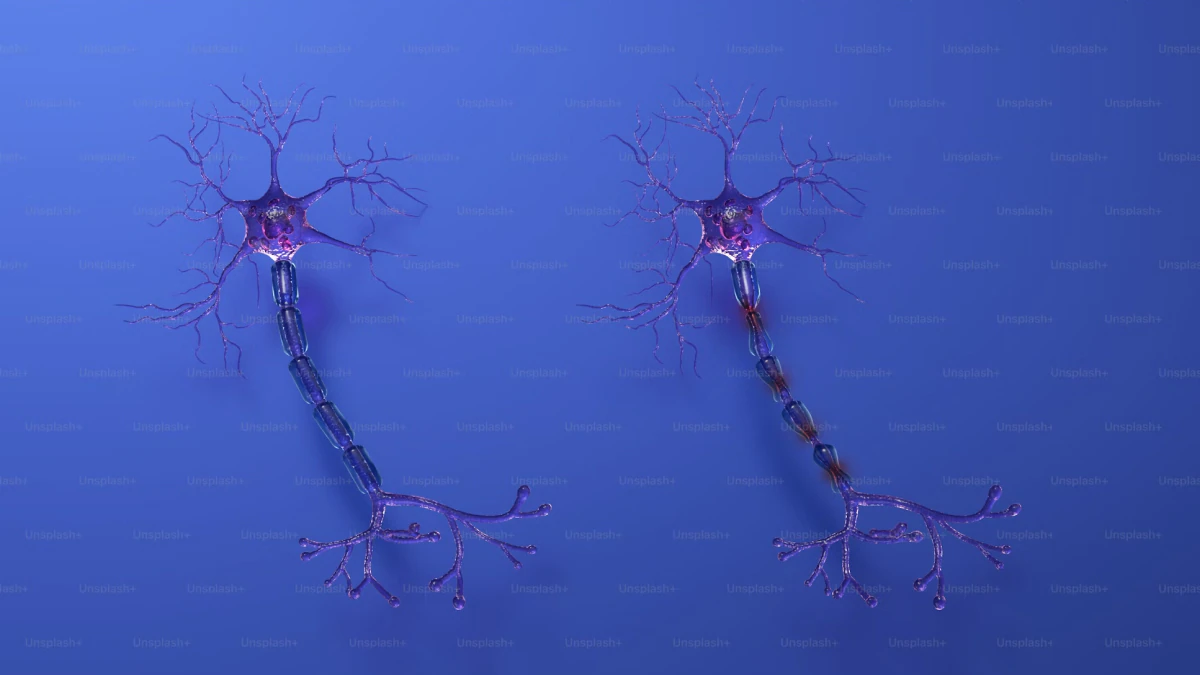
MIT researchers have unveiled a revolutionary technology: microscopic, wireless “cell-wearable” devices designed to wrap around individual neurons, potentially offering new therapies for neurological disorders like multiple sclerosis (MS). By creating synthetic myelin around damaged neurons, these wearables could restore function in areas where natural myelin is compromised—a hallmark of disorders like MS, which results in neuron degradation due to the loss of myelin, the protective layer surrounding neurons’ axons.
These tiny devices, made from a soft polymer called azobenzene, activate and roll around neuronal structures when exposed to light. The material’s unique properties enable these devices to wrap snugly around the complex shapes of neuronal structures, such as axons and dendrites, without causing damage. Since these wearables are battery-free and powered by light, thousands could be injected into the body, where they float and operate noninvasively to provide a synthetic myelin effect and stimulate neural activity.
The innovation, led by Deblina Sarkar of MIT’s Nano-Cybernetic Biotrek Lab, addresses a significant challenge: creating a device small and flexible enough to conform to the intricate shapes and curves of brain cells without harming them. This softness and adaptability make the devices especially promising for interfacing with fragile neurons. By adjusting the light intensity, researchers can control the size and shape of each device, allowing for a customizable fit around subcellular structures.
For fabrication, the team developed a scalable, cost-effective process that bypasses the need for cleanroom conditions, ensuring these tiny wearables could be produced in large quantities. Initial tests showed that these devices wrap securely around neurons and remain stable for days after activation. In trials on rat neurons, they demonstrated an ability to enhance electrical and metabolic function by forming a synthetic myelin layer.
Looking ahead, the research points toward applications beyond restoring neuron function. The devices can potentially be combined with advanced optoelectronic materials to further stimulate and monitor neural activity, suggesting future versions may contain built-in circuits for in-depth neuron monitoring and therapy. This breakthrough could lay the foundation for minimally invasive neural interfaces that restore function and protect neurons in a range of neurological conditions.
The work, published in Nature Communications Chemistry, is a significant leap forward in the field of bioelectronics, providing a new tool for neurobiological research and a novel approach to treating neurodegenerative diseases.